Distillation: the key to purify and concentrate liquids

Distillation is a laboratory technique used in the separation of miscible substances. The main objective of distillation is to separate a mixture of several components taking advantage of their different volatility, or to separate volatile materials from non-volatile ones.
The distillation process consists of heating a liquid until its most volatile components pass into the vapor phase and, later, cooling the vapor until these components are recovered in liquid form through a condensation process.
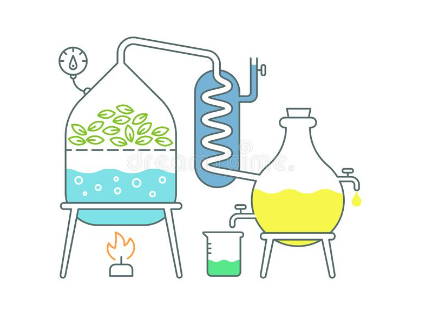
In general, the following elements are involved in the distillation process: Heat source, round bottom flask, fixed head, thermometer / boiling point temperature, condenser, cooling water, cooling water outlet, distillate / receiving flask, vacuum / gas inlet, fixed receiver, heat control, stirrer speed control, stirrer / heating plate, heating bath (oil / sand), stirring media, e.g. (picture), boiling chips or mechanical stirrer, cooling bath and distillation laboratory display.
types of distillation
Simple distillation: it is used when the mixture of liquid products to be distilled contains only one volatile substance, or when it contains more than one volatile substance, but the boiling point of the most volatile liquid differs from the boiling point of the other components in , at least, 80ºC.
Distillation at atmospheric pressure: that which is carried out at ambient pressure.
Distillation at reduced pressure: it consists of reducing the pressure in the distillation assembly in order to cause a decrease in the boiling point of the component to be distilled.
Fractional distillation: it is used when the mixture of liquid products to be distilled contains volatile substances of different boiling points with a difference between them of less than 80 ºC.
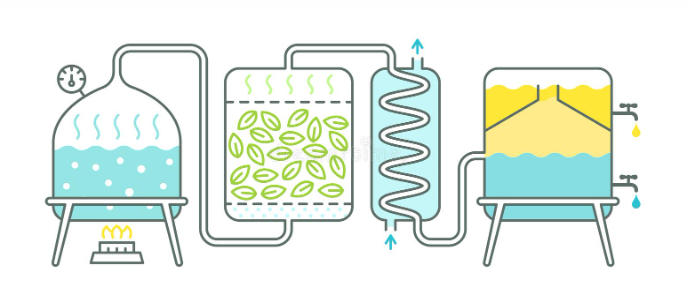
Steam distillation: allows the separation of substances insoluble in water and slightly volatile from other non-volatile products. It enables the purification or isolation of compounds with a high boiling point by distillation at low temperature (always below 100 ºC).
Distillation in a ball furnace: It consists of a vacuum distiller without dead volumes that is used for the separation between liquids or solids with a low melting point and polymeric substances or oils with a high boiling point.
Let’s see the next practice!





Responses