How much do we know about osmosis?

Osmosis is the phenomenon that occurs when two solutions with different concentrations are separated by a semi-permeable membrane and the solvent diffuses through the membrane from the lower concentration liquid to the higher one until the concentrations are balanced. This phenomenon occurs spontaneously without energy expenditure and is therefore a phenomenon of passive diffusion.
It is the mechanism whereby water passes through a semipermeable membrane from a hypotonic to a hypertonic solution. In other words, if, for example, we had two solutions of water and salt separated by a semi-permeable membrane (that is, one that only allows water to pass through); The water would move from the solution of lower concentration to the one of higher concentration without the need to provide energy thanks to the phenomenon of osmosis.
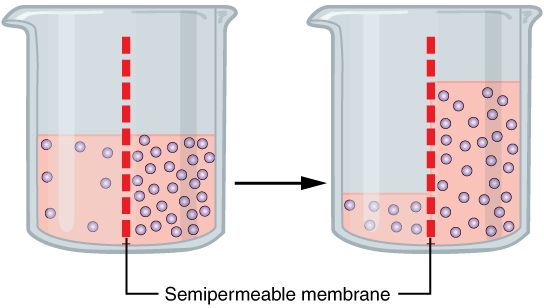
Aqueous media can have different concentrations of one or more solutes, the concentration of solvents and solutes (for example, water would be the solvent and salt would be the solute in the previous example) allows to classify aqueous media by comparison with respect to another in :
- Hypotonic medium: when the solute concentration is lower than the medium with which it is compared
- Hypertonic medium: when the solute concentration is higher than the medium with which it is compared.
- Isotonic medium: when both media have the same concentration.
The pressure exerted by the solvent (water) on the side of the membrane where there is a lower concentration towards the compartment with a higher concentration is called osmotic pressure. Continuing with the previous terminology, the pressure that occurs on the side of the membrane from the hypotonic medium to the hypertonic one is the osmotic pressure.
Let’s see the following experiment and put the osmosis effect into practice





Responses