Exploring the relationship between crystallization rate and crystal size

Crystallization rate is a fundamental property of materials science that refers to the rate at which crystals form from a liquid or solution. It is an important factor in controlling the size, shape, and quality of crystals, which may have significant implications for several fields, including pharmaceuticals, electronics, and materials engineering.
This can occur through several mechanisms, including nucleation and growth. Nucleation is the process of forming a small group of atoms or molecules, called a nucleus, which can then become a larger crystal; the nucleation rate is strongly influenced by factors such as temperature, concentration, and the presence of impurities. Once a nucleus has formed, the crystal can grow by adding more atoms or molecules to the crystal lattice.
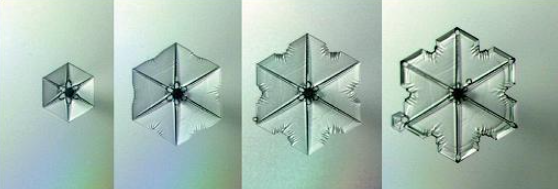
The rate at which crystals form is influenced by a variety of factors, including the rate of heat transfer, the concentration of the solute, and the presence of impurities or additives. In general, faster cooling rates or higher supersaturation levels will lead to faster nucleation rates and smaller crystal sizes, while slower cooling rates or lower supersaturation levels will lead to slower nucleation rates and larger crystal sizes.
Controlling the size and shape of crystals is an important consideration in many applications. For example, in the pharmaceutical industry, the size and shape of drug crystals can affect the rate of dissolution and absorption, which can affect the efficacy of the drug. In the electronics industry, the size and shape of semiconductor crystals can affect the electrical properties and performance of devices.



There are several techniques that can be used to control the size and shape of the crystals. One approach is to use additives or surfactants to modify nucleation and growth rates, which can result in the formation of smaller, more uniform crystals. Another approach is to use controlled heating or cooling rates to control the rate of nucleation and growth. In addition, techniques such as addition of seed crystals, sonication, and microwave irradiation can be used to promote crystal growth or induce crystal nucleation.
Let’s take a look at the following video that gives us more practical understanding about the rate of crystallization.





Responses