From fire to ice: the fascinating world of endothermic reactions

What are endothermic reactions? They are chemical reactions that need the supply of heat energy for them to occur. For the reactants to become products, these reactions absorb heat, which causes the products obtained to be left with higher energy levels than the initial reactants.
Enthalpy is a magnitude that defines the flow of thermal energy in chemical processes that occur at constant pressure. Furthermore, this magnitude represents the exchange of energy between a thermodynamic system and its surroundings; The variation of this magnitude in a chemical reaction is used to classify the reaction as endothermic or exothermic.
These reactions are commonly used in the chemical ice and refrigeration industry, since they can be generated in controlled environments to remove heat from the environment or from other substances. Some of its applications were replaced with the cold generated by the cooling equipment.
Examples of endothermic reactions
Some examples of endothermic reactions are:
Ozone production in the atmosphere. This reaction is driven by ultraviolet radiation from the Sun, the oxygen (O2) molecules are converted into ozone (O3), absorbing energy from said radiation in the process.

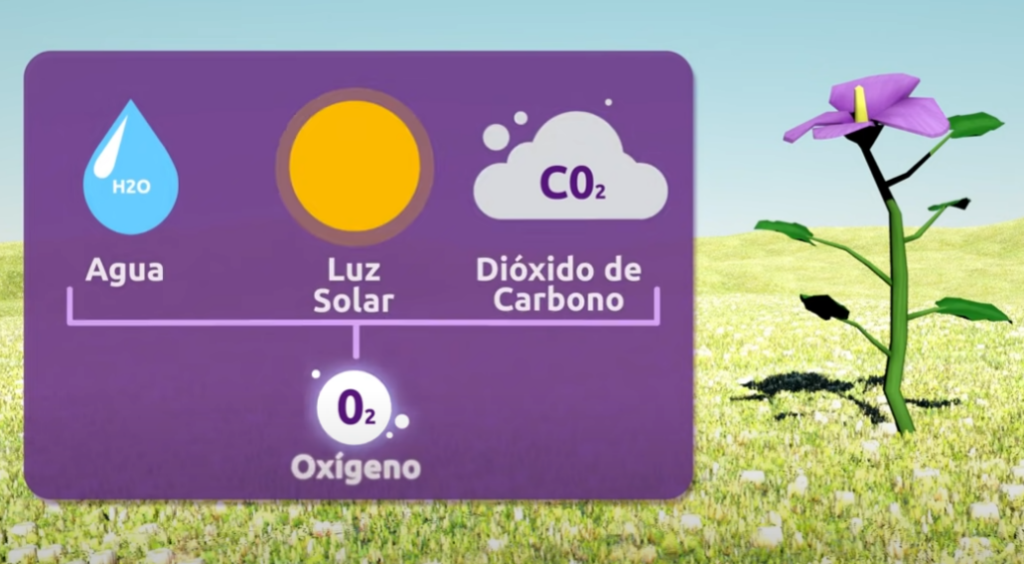
Water electrolysis. To separate the hydrogen (H) and oxygen (O) that make up water (H2O) it is necessary to add electrical energy in a procedure known as electrolysis, in which both types of atoms respond to the poles generated by the added electrical current, their chemical bond is broken and energy is consumed.
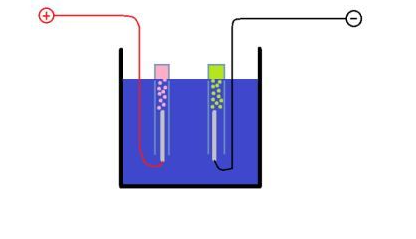
Photosynthesis. The plant nutrition process occurs through a series of chemical reactions that decompose environmental carbon dioxide (CO2) in the presence of water and sunlight; this series of reactions needs to consume energy to occur.
Obtaining iron sulfide (II). This compound is obtained in a laboratory after reacting iron and sulfur. For this reaction to occur it is necessary to supply heat energy using a burner (or a boiler if it is industrial conditions).

Let’s see the next practice!




Responses