Do you know what is electrolysis?

Electrolysis is the process in which the elements of a compound are separated by the application of electricity, in other words, electrical energy is transformed into chemical energy. The chemical process called electrolysis has gained a lot of prominence in recent years, since it is key in the green hydrogen production process, however, it is not a novelty, it has been used for decades in many other applications.
For the electrochemical process called electrolysis to occur, two elements are necessary, in addition to the electric current that will make the non-spontaneous chemical reaction possible: the electrodes and the electrolyte. The electrolyte is an aqueous or saline solution in which the electrodes (anode and cathode) are submerged; the set of all these elements is called an electrolysis cell. The electrolyte allows the transfer of ions between the anode and the cathode when an electric current is applied.
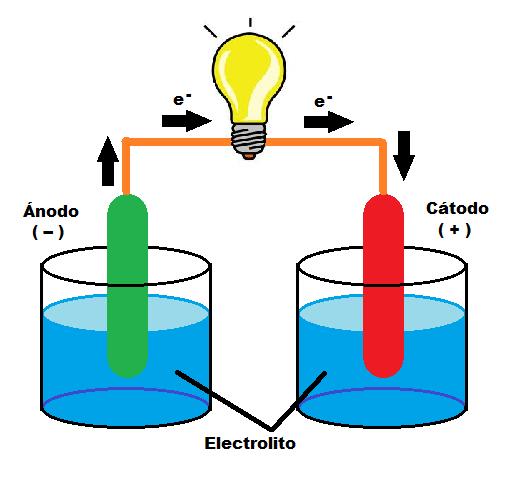
How does electrolysis work? The electrolyte is melted or dissolved in a solvent so that the separation of ions occurs, a process called ionization. A direct electric current is then applied through the electrodes (anode and cathode) immersed in the solution and connected to a power supply. Electron transfer occurs, positive ions or cations go to the cathode and negative ions or anions go to the anode.
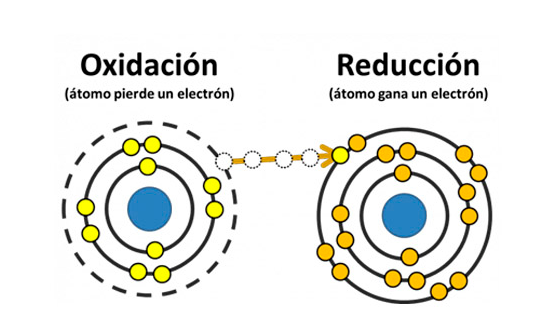
As a result of this chemical reaction, other substances are produced, the anions give up electrons to the anode (+) and the cations take electrons from the cathode (-). In this way the oxidation-reduction reaction is completed through the contribution of electricity as a necessary energy source for it. Upon completion of the process, oxygen and hydrogen are given off, as well as other materials if non-distilled water has been used.
Some important aspects to take into account when carrying out the electrolysis process is that the electrodes should never come into direct contact, because in that case the process is not completed and the battery will overheat and burn. In addition, the applied electric current must always be continuous, that is, never through a plug, but through a battery or power adapter and finally, it is important that the gases released (oxygen and hydrogen) do not come into contact with each other, since otherwise they would produce water again.
The electrolysis process is used to produce green hydrogen, as well as in batteries, including those for electric cars, and is also used in the production of materials and chemical compounds such as aluminum, sodium, hydrochloric acid or hypochlorite (bleach), among many others. It gives way to electrometallurgy, which is a process to separate pure metal from other compounds, it is also used for the anodizing method, which serves to increase the thickness of the natural oxide layer on the surface of metal parts, and works to perform electroplating, which is the creation of a protective film to protect a metal from corrosion.
Let’s do the following practice and see how electrolysis works



Responses