Oxidation Nation: A Deep Dive Into Redox Reactions
In chemistry, chemical reactions in which an exchange of electrons occurs between the atoms or molecules involved are known as redox reactions, oxidation-reduction reactions or reduction-oxidation reactions. This exchange is reflected in the change of oxidation state of the reactants, the reactant that gives up electrons undergoes oxidation and the one that receives them, reduction.
The oxidation state indicates the number of electrons that an atom of a chemical element gives up or accepts when it is part of a chemical reaction. It can also be interpreted as the supposed electrical charge that a certain atom would have if all its bonds with other atoms were completely ionic, it is also called the oxidation or valence number.
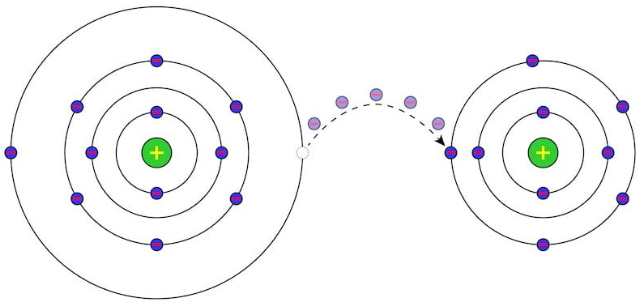
The oxidation state is expressed in whole numbers, the oxidation state being zero for neutral elements. Así, puede tomar valores positivos o negativos dependiendo del tipo de átomo y de la reacción donde participe. On the other hand, some atoms have variable oxidation states depending on the reaction in which they are involved.
In this way, in every redox reaction there are two types of reactants, one that gives up electrons and the other that accepts them:
Un agente oxidante. It is the atom that captures the electrons, in this sense, its initial oxidation state decreases, and a reduction is experienced. In this way, it increases its negative electrical charge by gaining electrons.
A reducing agent. It is the atom that gives up the electrons and increases its initial oxidation state, undergoing oxidation. In this way, it increases its positive electrical charge by giving up electrons.

Some chemical elements can be oxidized and reduced at the same time. A estos elementos se les llaman anfolitos y el proceso en el cual sucede esto se denomina anfolización.
Redox reactions are one of the most common chemical reactions in the universe, as they are part of the processes of photosynthesis in plants and respiration in animals, which allow the continuity of life.
Let’s check out the next video that includes a little experiment of a redox reaction!



Responses