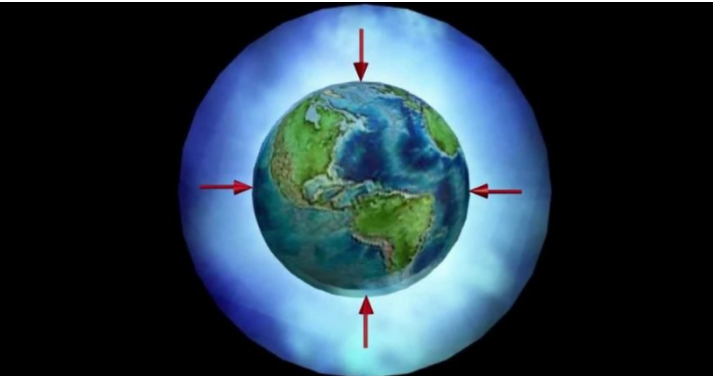
In itself, what is atmospheric pressure?

Atmospheric pressure or barometric pressure is the force exerted by the set of mixed gases that make up the atmosphere, on the earth’s surface and the elements that are on it. Said force is given per surface unit, that is, it is equivalent to the weight of the air column that extends from a point on the Earth’s surface, to the upper limits of the atmosphere, that is, air is a gas what weighs; Atmospheric pressure is nothing more than the weight of air per unit area.
Atmospheric pressure and its variations over a period of time are common data in the study of atmospheric climate, however, air varies in density as it moves away from the ground and is also affected by temperature, so It is usually not easy to calculate the atmospheric pressure of a given point with certainty.
In antiquity, the mere idea of atmospheric pressure was unknown, and its daily effects, such as the practical limitation of the rise of certain materials, was understood as evidence of the horror vacuis, that is, the “terror of the vacuum” that nature manifested, since it was thought that air was weightless and rose on its own.
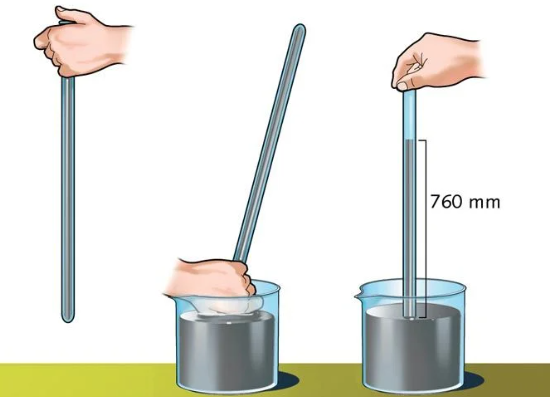
This was the case until, in 1643, the weight of air was discovered by the Italian physicist Evangelista Torricelli (1608-1647), through the first experiments that led to the creation of the barometer. His most famous experiment consisted of comparing the behavior of mercury and water when introduced into a curved tube at the end, known today as a Torricelli tube.
His experiences helped the French polymath Blaise Pascal (1623-1662) to measure the weight of atmospheric air in different geographical locations and different altitudes, such as the top of the Puy-de-Dôme volcano in southern France. But it was not until 1654, thanks to the experiments with the Magburg hemispheres of the German physicist Otto von Guericke (1602-1686), that the existence of atmospheric pressure was publicly demonstrated.
Atmospheric pressure is, above all, a form of pressure, which is why it is measured in the International System of Units (SI) in Pascals (Pa), a unit that pays tribute to the French physicist and is understood as the pressure exerted by a force of 1 newton (N) on a surface of 1 square meter (m2) normal to it, that is: Pa = kg/m.s2.

To measure the atmospheric pressure of a certain place, we need a device called a barometer. Its fundamental principle, which reproduces Torricelli’s experiences in the 17th century, consists of a column of liquid (usually mercury) introduced into a tube whose upper portion is closed. In this way, the weight of the air in the atmosphere exerts more or less force on the liquid, forcing it to remain inside the tube to a certain point, equivalent to the force itself received.
Let’s perform the following quiz and test our knowledge about atmospheric pressure




Responses