Precipitation reactions: separating the soluble from the insoluble

What are precipitation reactions? The precipitation reaction is the reaction that occurs between two substances in an aqueous medium, where a product is formed that does not dissolve in water. This reaction can be easily identified by the formation of a solid that precipitates, therefore this solid is also called a precipitate.
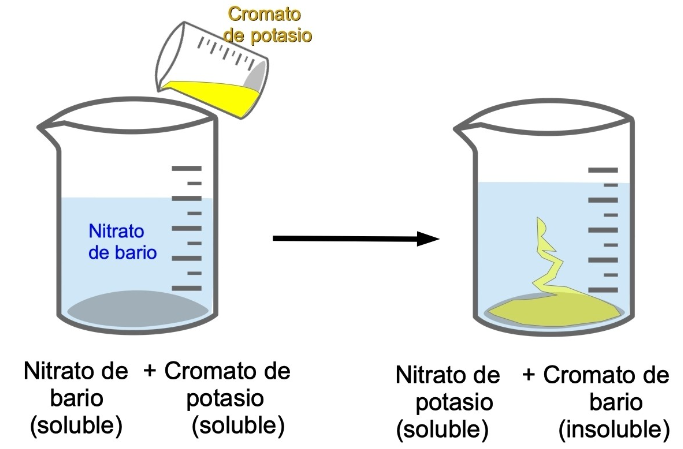
Typically, precipitation reactions occur with ionic compounds, that is, compounds that break down into their constituent ions in water. For example, potassium chromate separates into CrO2-4 chromate ion and potassium ion, while barium nitrate separates into nitrate ion and barium ion.
Precipitate Formation Rules
To recognize a precipitation reaction, we must predict whether the product that forms is soluble or insoluble in water. For this, there are the following rules:
- The solid compound must have zero charge. Therefore, two negatively charged ions or anions cannot combine, nor can two positively charged ions or cations.
- For the compound to have no charge, a cation and an anion must combine to balance their charges with each other.
- Most nitrate salts are soluble.
- Most of the salts containing alkali metal ions and the ammonium ion are soluble.
- Most salts with chlorine, bromine, or iodine are soluble, except when silver, lead, and mercury ions are present.
- Most sulfate salts are soluble, except for barium sulfate, lead sulfate, mercury sulfate, and calcium sulfate.
- The soluble hydroxides are sodium hydroxide and potassium hydroxide. The moderately soluble compounds are barium hydroxide, strontium hydroxide and calcium hydroxide.
- Most sulfides, carbonates, chromates, and phosphates are slightly soluble, with the exception of salts with rule 4 ions.


Let’s take a look at the next experiment and if you can, let’s get down to business!




Responses