What is Coulomb’s Law?
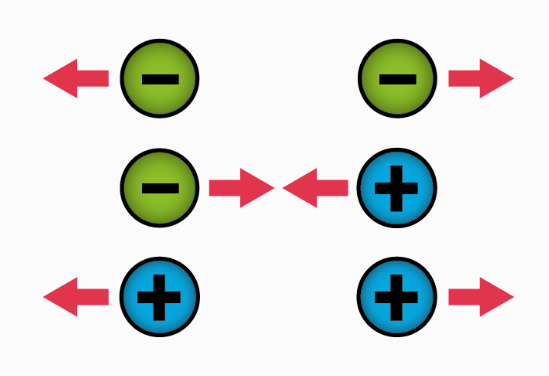
Coulomb’s law is used in the area of physics to calculate the electrical force that acts between two charges at rest. From this law it can be predicted what will be the electrostatic force of attraction or repulsion between two particles according to their electric charge and the distance between them.
Coulomb’s law owes its name to the French physicist Charles-Augustin de Coulomb, who in 1875 enunciated this law, and which constitutes the basis of electrostatics:
The magnitude of each of the electrical forces with which two point charges at rest interact is directly proportional to the product of the magnitude of both charges and inversely proportional to the square of the distance that separates them and has the direction of the line that joins them. The force is repulsive if the charges are of the same sign, and attractive if they are of the opposite sign.
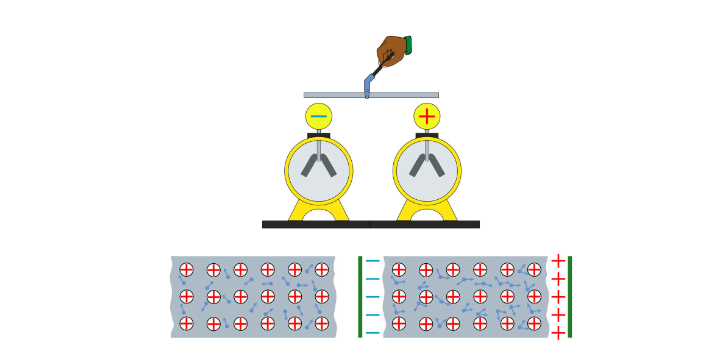
Coulomb managed to determine the properties of the electrostatic force by developing a torsion balance as a study instrument, which consisted of a bar that hung on a fiber with the ability to twist and return to its initial position.
In this way, Coulomb could measure the force that was exerted on a point of the bar by placing several charged spheres at different distances in order to measure the force of attraction or repulsion as the bar rotated.
Observamos el siguiente experimento y realicemos juntos para aprender un poco más sobre este concepto




Responses